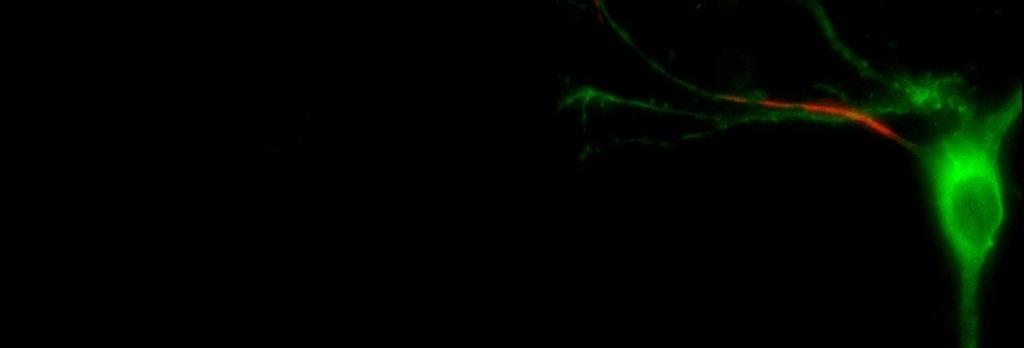
Your brain contains billions of
inter-connected cells, or neurons, which work by sending electrical
signals from one to another. These
electrical events are vital in adult brains, but they're also a feature of the
young developing nervous system. What's
more, changing the electrical activity that occurs in the developing brain can
have dramatic effects on a neuron's identity, its position, its shape, and its
connections with other cells. In other
words, electrical signals are crucial for normal brain development.
Our lab is interested in just how
electrical activity influences the development of the brain, with special
emphasis on two inter-connected processes:
1) The first involves a highly specialised
part of the neuron. Brain cells have a
very distinct shape that you're probably familiar with, but what you might not
know is that different parts of a neuron are designed to carry out certain
specific tasks in processing electrical signals. In simple terms, the large spherical bulge in
the middle of the cell is known as the cell body, or soma. It contains all of the 'housekeeping'
machinery needed to keep the cell healthy and functioning. Sticking out from the soma and spreading to
look something like the branches of a tree are processes called dendrites,
which act primarily as the cell's input-receiving area – they listen in to
signals being sent to them from other neurons.
Once they arrive in the dendrites, these signals spread, going further
the stronger they are. If they are
strong enough, they might spread into the axon, which is long,
thin, and extends from the soma. This is the output structure of the neuron,
using input coming in from the dendrites and soma to generate big electrical
signals called action potentials, which then travel to the end of the
axon (the axon terminals) and there start to influence the activity of
other brain cells.
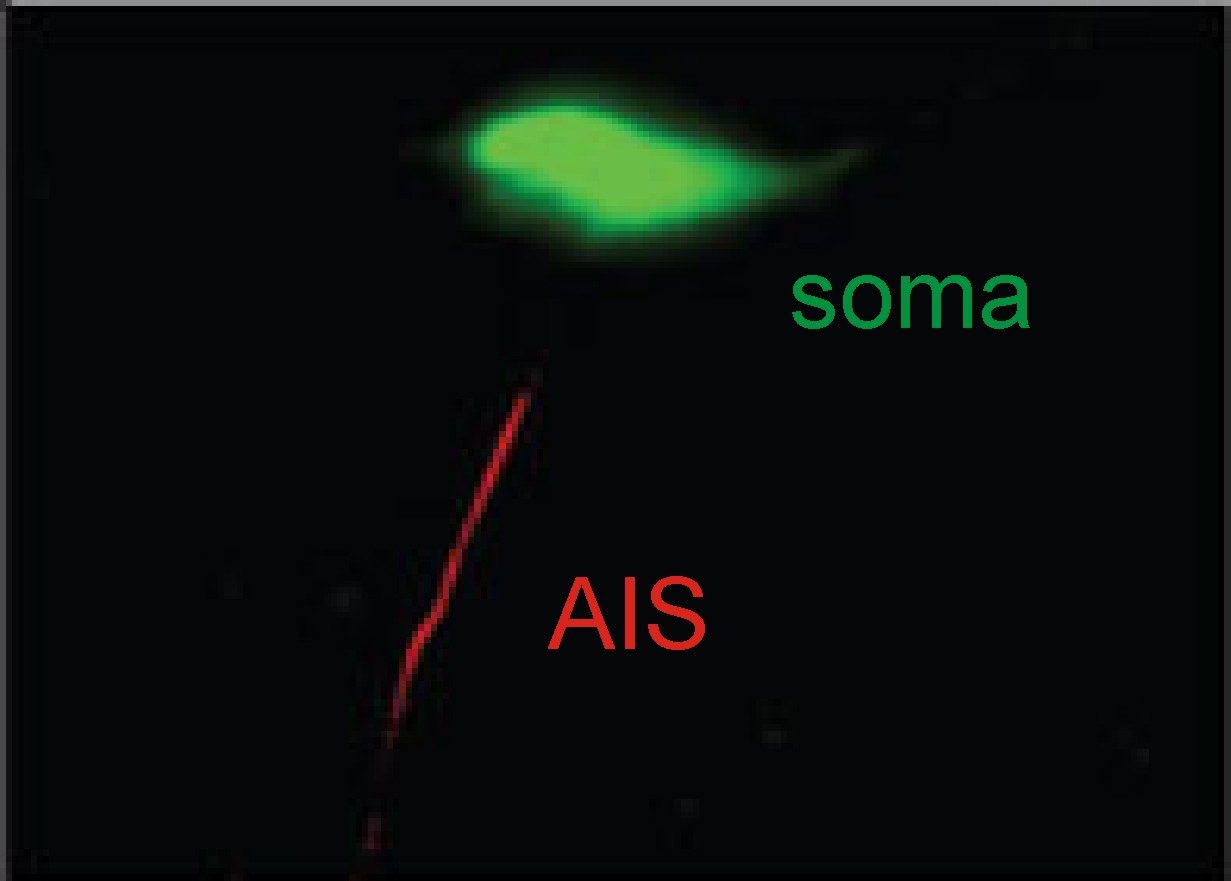 We're interested in a particular 'hotspot'
near the start of the axon, called the axon initial segment, or AIS. This is the part of a neuron which is most
sensitive to electrical activity – it needs the lowest amount of input in order
to generate an action potential, which means that it is the site where action
potental signals invariably start.
Despite this crucial role in kick-starting communication between brain
cells, scientists actually know surprisingly little about the AIS, and even
less about how it develops and changes over time.
Our lab has published evidence that the AIS
can change a lot in response to alterations in the ongoing electrical activity
of brain cells – when we made the cells more active, we saw the location of the
AIS change strikingly, with the whole structure shifting itself along the axon
away from the cell body (you can read good summaries of this work on the Nature
website and the Alzforum
website, or listen to us talking about it on the Nature NeuroPod
podcast (12.49 min) if you're keen).
Because the electical signals to a cell come through the dendrites and
soma before reaching the AIS (see above), this means that the most excitable
part of the neuron is actually 'running away' from its input, which acts to
make the cell as a whole less excitable.
In other words, whereas before a certain minimum amount of input would
make the neuron produce an action potential, after the AIS has moved away from
the soma the cell would need to receive stronger input before an action
potential was generated. We think this
is a feedback mechanism a bit like a thermostat – when the neuron is too
active, it moves the AIS away from the soma, which then brings its activity
back to normal.
Current work in the lab is focusing on
exactly how this AIS movement occurs – what are the mechanisms which shift this
portion of the cell around, and how can we influence these so we can have good
control over the activity levels of individual brain cells? Answers to these questions might help a
little in the search for treatments for brain disorders where electrical
activity levels are abnormal, such as epilepsy.
 2) The second main interest of the lab is a
part of the brain called the olfactory bulb. This is the first brain region to receive
information about smells from the nose.
It processes that information and then passes it onto other parts of the
olfactory system where our perceptions of smell and flavour are created. We're interested in the olfactory bulb
because we're fascinated by how our sense of smell works, and also because it
has some unusual properties which make it a great model system to study basic
processes of maturation in the brain.
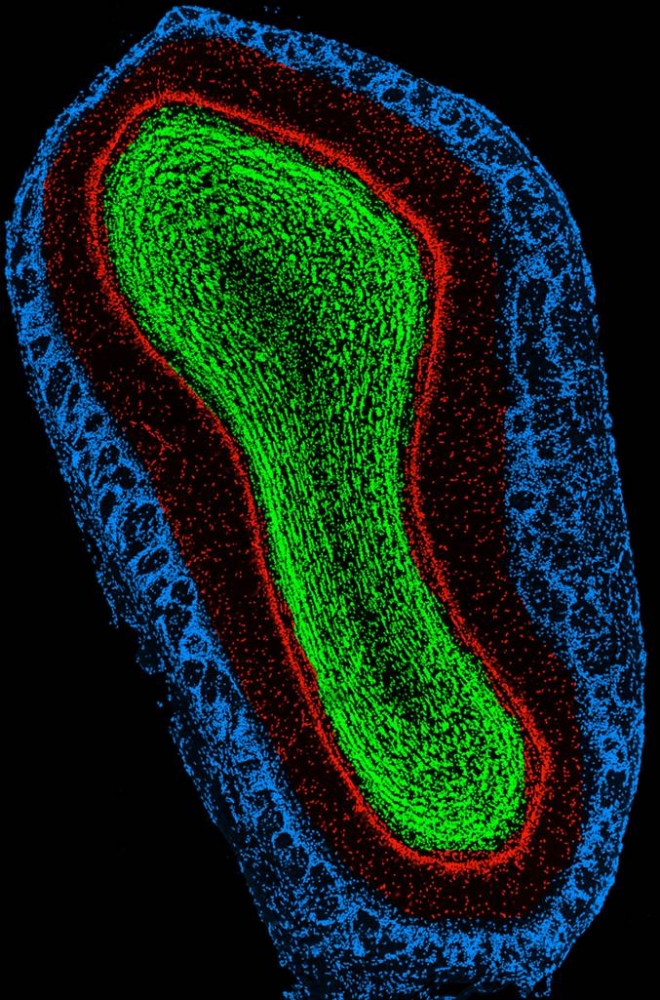 Unlike most other areas of the brain, the
olfactory bulb continually changes its cells throughout life, in a process
known as adult neurogenesis.
Whereas most of the neurons in your brain right now were born before you
were, and won't be replaced if you lose them, certain types of cell in your
olfactory bulb were born very recently, and might be replaced in a couple of
months by freshly-born neurons generated through adult neurogenesis. Many scientists are actively investigating
just why this one area of the brain continues to receive new neurons throughout
life, but we're intrigued by a different question thrown up by adult
neurogenesis, which is how exactly the adult-born cells manage to do their job. Think about it – growing a brain
where all the neurons are immature and need to set themselves up with all the
right interconnections is hard enough, but how does a newly-generated cell squeeze
its way into a network of interconnected neurons that are already established
and doing their smell-processing work, without messing that network up?
Cross section of the olfactory bulb
We, along with other scientists in the
field, think that the answer lies in the electrical events experienced by
adult-born olfactory bulb cells. We know
that ongoing electrical activity can influence many aspects of brain
development, and we know that levels of electrical activity can influence some
aspects of maturation in the adult olfactory bulb. However, there are many maturational
processes in these cells that we don't understand at present, among them the
formation of the AIS in adult-born olfactory bulb neurons, and the way in which
the AIS in these cells can respond to changes in electrical activity.
These are both questions that we are
actively pursuing in the lab right now.
We hope that answering them, along with other unsolved issues about
neuronal maturation in adult neurogenesis, might one day help to inform
treatments trying to replace human cells lost through injury or disease. If we're trying to replace old cells with new
cells, why not take a few hints from the way the brain does it all on its own?
An adult-born neuron in the olfactory bulb
|